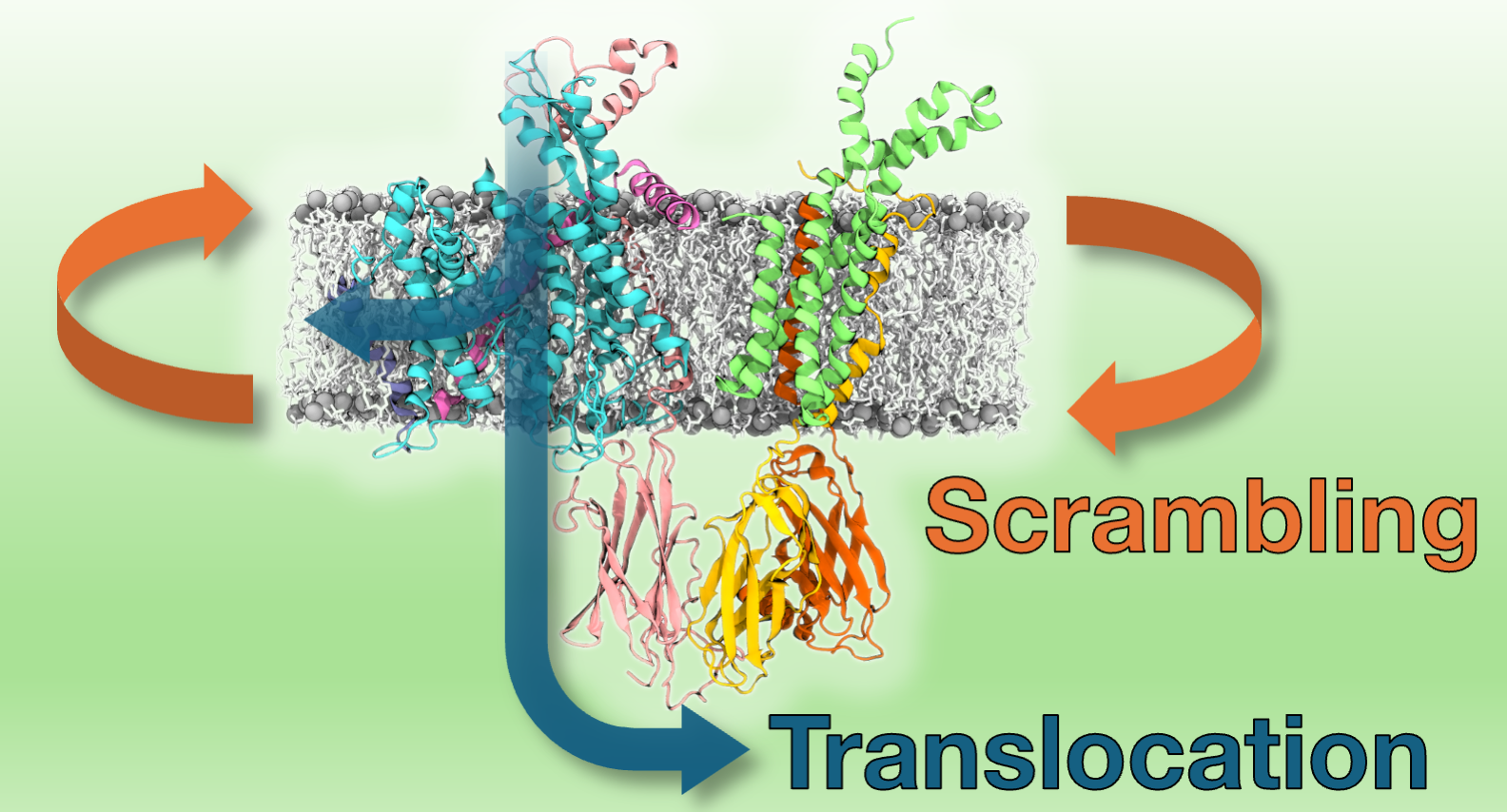
Using fluorescence experiments, we demonstrated lipid scrambling by our isolated and reconstituted Sec61 translocon complexes. We then used coarse-grained molecular dynamics simulations to pinpoint this scrambling activity to not only the Sec61 complex, but also to the trimeric transmembrane helix bundle of the translocon-associated protein (TRAP) complex. This scrambling by TRAP is compatible with our experiments performed with Sec61 inhibitors, in which scrambling activity by the translocon complexes was still observed. Further analyses of our simulations allowed to study the effect of membrane thinning on scrambling, its lipid selectivity, as well as the thermodynamics and kinetics of the process.
A great interdisciplinary collaboration with the groups of Ville Paavilainen (University of Helsinki) and Radek Šachl (J. Heyrovsky Institute of Physical Chemistry, Czech Academy of Sciences), as well as with Denys Biriukov (CEITEC Brno).
Link to paper in the Journal of the American Chemical Society: