The main focus of our research is to develop systematically administered, target-seeking therapeutic molecules for the regenerative medicine. This would increase their therapeutic value in their target (“diseased”) organ while simultaneously reducing the harmful side-effects in the normal tissues.
Our approach is based on the fact that the blood vessels in each tissue have a unique molecular fingerprint that is specific for that given tissue, i.e. vascular “Zip code”. Furthermore, human diseases also cause molecular changes (induced expression of specific cell surface markers) in the blood vessels supplying the involved organ. For example, angiogenic blood vessels in tumors and in regenerating tissues express distinctive markers that are not present in vessels of normal tissues. These specific cell surface markers, in turn, can be specifically targeted by vascular homing peptides that home to their target organ when administered systemically. We have identified vascular homing peptides that specifically target the angiogenic blood vessels and the vasculature inflammatory diseases. Utilizing these target-tissue specific homing peptides, we have devised two novel delivery mechanisms: A) building multi-functional fusion-proteins consisting of separate domains for targeting (and tissue penetration) and therapeutic effects (Fig. 1; Järvinen & Ruoslahti 2010)
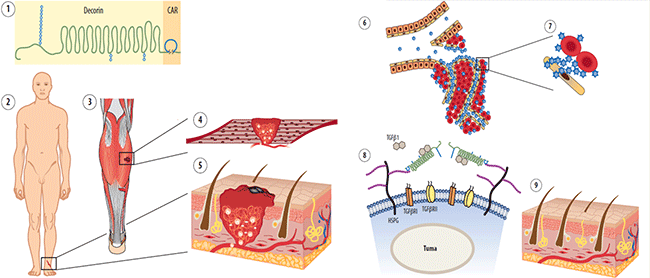
Fig. 1. Schematic presentation of the mechanism of action of multi-functional therapeutic molecule CAR-decorin.
and CAR-decorin (1) is a systemically administered, target-seeking biotherapeutic that inhibits scar formation. The injury can happen in any organ of the body (2, 3) (or multiple organs simultaneously) and systemically administered vascular homing peptide CAR can find there through blood stream by targeting angiogenic vasculature forming at the site of the injury (4, 5). CAR-decorin binds to its receptor specific for the vasculature of the injured tissues and extravasates into surrounding tissue (6), where it binds to its receptor on the cell surface of the scar producing fibroblasts (7). The CAR binding to the HSPGs provides ideal docking for the therapeutic part of the molecule, decorin, for binding and neutralization of its molecular targets; TGF-b1 and -b2, the growth factors responsible for scar formation (8). The clinical outcome is reduced scarring (9).
B) recently published revolutionary tissue-penetrating transport pathway called “bystander effect”, to target the drugs without conjugating them to the delivery vehicle (Fig 2). The “bystander effect” provides the advantage of promoting delivery of a drug to its target tissue without the need of coupling of the drug to the peptide required in conventional drug targeting (Fig. 2).
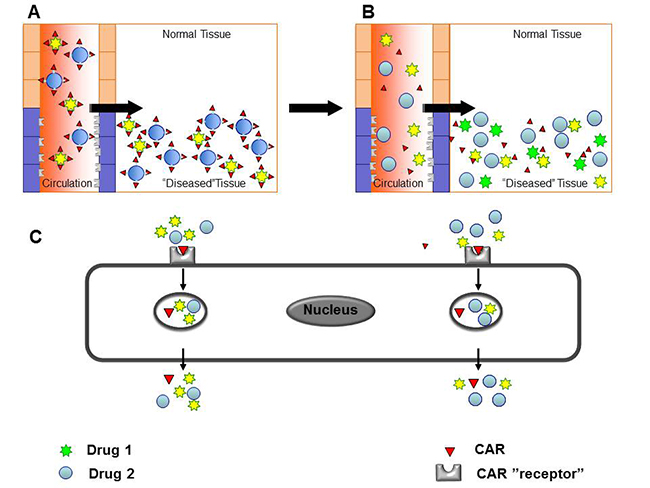
Fig. 2. Drug delivery by the cell penetrating, vascular homing peptides induced “bystander effect”
A) Targeting domain and the therapeutic molecule are fused together for tissue-specificity in conventional, conjugated delivery. B – C) Compounds co-injected with tissue-penetrating peptides are transported across the vessel wall and through tissue together with the cell penetrating, vascular homing peptides.
More information:
Selected publications
Maldonado H, Savage BD, May U, Vähätupa M, Barker H, Badiani RK, Wolanska, KI, Turner CML, Pemmari T, Ketomäki T, Prince S, Humphries MJ, Ruoslahti E, Morgan MR, Järvinen TAH: Systemically administered wound homing peptide accelerates wound healing by modulating syndecan-4 function. Nat Comm, 10.1038/s41467-023-43848-1, 2023. (https://www.nature.com/articles/s41467-023-43848-1)
Pemmari T, Ivanova L, May U, Lingasamy P, Pasternack A, Prince S, Ritvos O, Makkapati S, Teesalu T, Cairo MS, Järvinen TAH, Liao Y: Exposed CendR domain in homing peptide yields skin-targeted therapeutic in epidermolysis bullosa. Mol Ther 28:1833-1845, 2020 (https://www.sciencedirect.com/science/article/pii/S1525001620302513?via%3Dihub)
De Rossi G, Vähätupa M, Cristante E, Liyanage SE, May U, Pellinen L, Uusitalo-Järvinen H, Bainbridge JW, Järvinen TAH, Whiteford JR. Pathological angiogenesis requires Syndecan-4 for efficient VEGFA-induced VE-Cadherin internalization. Arteriosclerosis, Thrombosis and Vascular Biology (ATVB) 41:1374-1389, 2021. (https://www.ahajournals.org/doi/10.1161/ATVBAHA.121.315941)
Pemmari T, Prince S, Wiss N, Kõiv K, May U, Sudakov A, Molder T, Caro FM, Lehtonen S, Uusitalo-Järvinen H, Teesalu T, Järvinen TAH: Screening of homing and tissue-penetrating peptides by microdialysis and in vivo phage display. Life Sci Alliance, 8: e202201490, 2025. (DOI: 10.26508/lsa.202201490) (https://www.life-science-alliance.org/content/8/5/e202201490)
Järvinen TAH. Neovascularization and tendinopathy: from eradication to stabilization? Editorial. Br J Sports Med 54:1-2, 2020 (https://bjsm.bmj.com/content/54/1/1.long)
Barker H, Aaltonen L, Pan P, Vähätupa M, Kaipiainen P, May U, Prince S, Uusitalo-Järvinen H, Waheed A, Pastoreková S, Sly WS, Parkkila S, Järvinen TAH. Role of carbonic anhydrases in wound healing. Exp Mol Med, 49:e334, 2017. (12.9) (https://www.nature.com/articles/emm201760)
