Publications
Selected publications
SpyTag/SpyCatcher display of influenza M2e peptide on norovirus-like particle provides stronger immunization than direct genetic fusion
A hexavalent Coxsackievirus B vaccine is highly immunogenic and has a strong protective capacity in mice and nonhuman primates
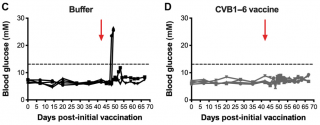 Here we describe the generation and preclinical testing of a novel hexavalent vaccine targeting the six known CVB serotypes. We show that the vaccine has an excellent safety profile in murine models and nonhuman primates and that it induces strong neutralizing antibody responses to the six serotypes in both species without an adjuvant. We also demonstrate that the vaccine provides immunity against acute CVB infections in mice, including CVB infections known to cause virus-induced myocarditis. In addition, it blocks CVB-induced diabetes in a genetically permissive mouse model. Our preclinical proof-of-concept studies demonstrate the successful generation of a promising hexavalent CVB vaccine with high immunogenicity capable of preventing CVB-induced diseases.
Here we describe the generation and preclinical testing of a novel hexavalent vaccine targeting the six known CVB serotypes. We show that the vaccine has an excellent safety profile in murine models and nonhuman primates and that it induces strong neutralizing antibody responses to the six serotypes in both species without an adjuvant. We also demonstrate that the vaccine provides immunity against acute CVB infections in mice, including CVB infections known to cause virus-induced myocarditis. In addition, it blocks CVB-induced diabetes in a genetically permissive mouse model. Our preclinical proof-of-concept studies demonstrate the successful generation of a promising hexavalent CVB vaccine with high immunogenicity capable of preventing CVB-induced diseases.
Structural Insight into CVB3-VLP Non-Adjuvanted Vaccine
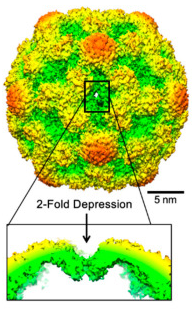 Here, we describe the production of CVB3-VLPs with enhanced production yield and purity using an improved purification method consisting of tangential flow filtration and ion exchange chromatography, which is compatible with industrial scale production. We also resolved the CVB3-VLP structure by Cryo-Electron Microscopy imaging and single particle reconstruction. The structural and immunogenic data presented here indicate the potential of this improved methodology to produce highly immunogenic enterovirus VLP-vaccines in the future.
Here, we describe the production of CVB3-VLPs with enhanced production yield and purity using an improved purification method consisting of tangential flow filtration and ion exchange chromatography, which is compatible with industrial scale production. We also resolved the CVB3-VLP structure by Cryo-Electron Microscopy imaging and single particle reconstruction. The structural and immunogenic data presented here indicate the potential of this improved methodology to produce highly immunogenic enterovirus VLP-vaccines in the future.
Formalin treatment increases the stability and immunogenicity of coxsackievirus B1 VLP vaccine
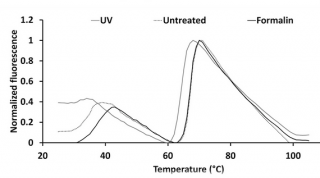 Type B Coxsackieviruses (CVBs) are a common cause of acute and chronic myocarditis, dilated cardiomyopathy and aseptic meningitis. However, no CVB-vaccines are available for human use. We have previously produced virus-like particles (VLPs) for CVB3 with a baculovirus-insect cell production system. Here we have explored the potential of a VLP-based vaccine targeting CVB1 and describe the production of CVB1-VLPs with a scalable VLP purification method.
Type B Coxsackieviruses (CVBs) are a common cause of acute and chronic myocarditis, dilated cardiomyopathy and aseptic meningitis. However, no CVB-vaccines are available for human use. We have previously produced virus-like particles (VLPs) for CVB3 with a baculovirus-insect cell production system. Here we have explored the potential of a VLP-based vaccine targeting CVB1 and describe the production of CVB1-VLPs with a scalable VLP purification method.
Modular vaccine platform based on the norovirus-like particle
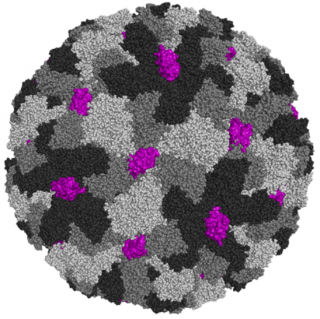 SpyTagged noro-VLP was expressed with high efficiency in insect cells and purified using industrially scalable methods. Like the native noro-VLP, SpyTagged noro-VLP is stable for months when refrigerated in a physiological buffer. The modular noro-VLP vaccine platform presented here offers a rapid, convenient and safe method to present various soluble protein antigens to the immune system for vaccination and antibody production purposes.
SpyTagged noro-VLP was expressed with high efficiency in insect cells and purified using industrially scalable methods. Like the native noro-VLP, SpyTagged noro-VLP is stable for months when refrigerated in a physiological buffer. The modular noro-VLP vaccine platform presented here offers a rapid, convenient and safe method to present various soluble protein antigens to the immune system for vaccination and antibody production purposes.
