Structural biology of Janus kinases
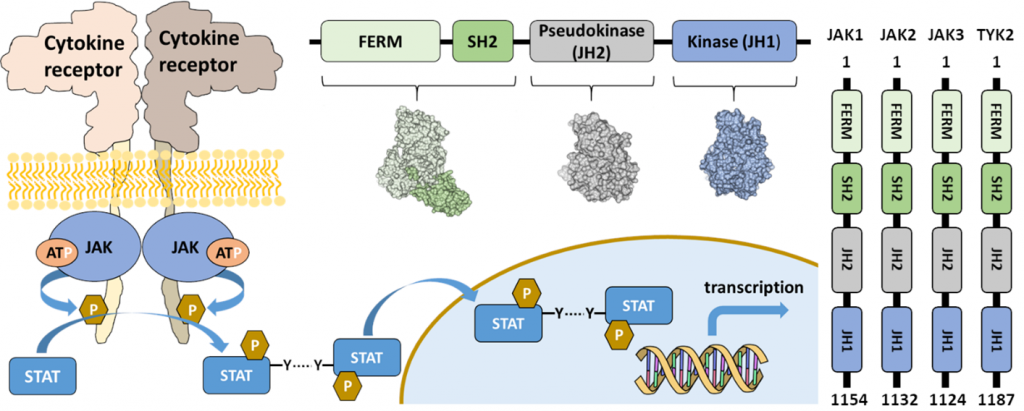
Janus kinases (JAKs) are a family of four non-receptor tyrosine kinases possessing a unique dual kinase domain arrangement; an inactive pseudokinase domain followed by an active tyrosine kinase domain. The activity of JAKs is regulated via an interplay between the pseudokinase and kinase domains and several pathogenic disease mutations disrupt this regulation leading to autoimmune and malignant diseases. We utilize structural biology, including X-ray crystallography, NSEM, and cryo-EM combined with computational methods to study the molecular details of JAK activation in disease.
Assay development
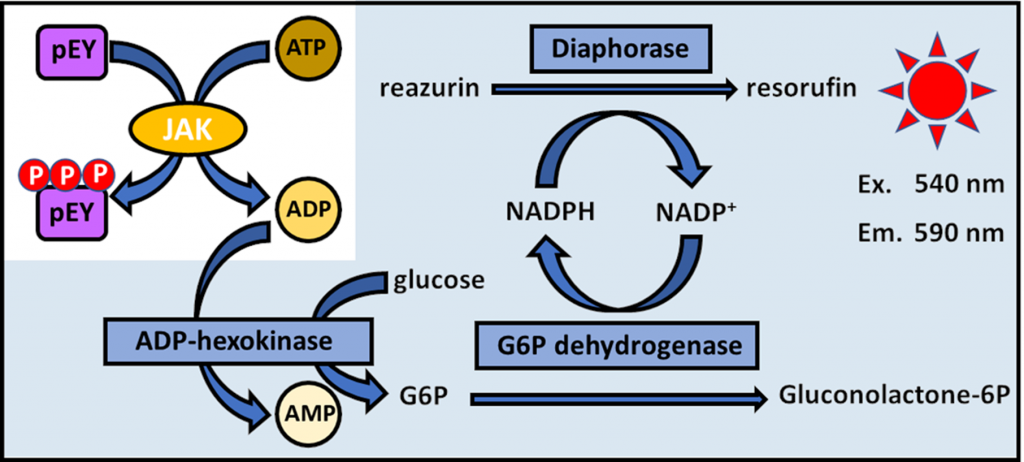
We develop inhibitor screening assays against protein kinases, especially focusing on the discovery of non-ATP competitive inhibitors. We are interested in cell-based and in vitro assays that can be used in medium to high-throughput fashion. We utilize these assays for the identification of new kinase inhibitor scaffolds for further optimization.
Drug discovery
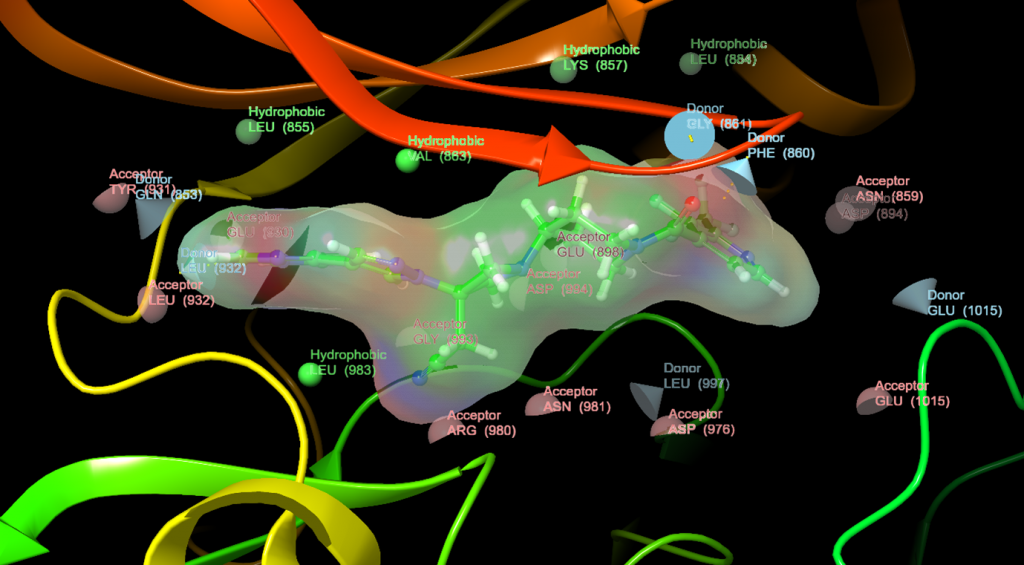
We utilize structure-based design to improve screening hits into more potent and selective compounds. Structure determination of kinase-inhibitor complexes with X-ray crystallography combined with computational discovery and design of new molecules form the basis of our discovery process. Current projects focus on kinases involved in the pathogenesis of blood cancers and mycobacterial infections.
