Authors: Oskari Kulta & Susanna Narkilahti
Introduction: The Role of Bioethics in Medicine, Research and Biotechnology
Bioethics is the study of ethical issues arising from advances in biology, medicine, and technology. It examines how scientific progress intersects with moral values, human rights, and social responsibility. In medicine, biomedical research, and biomedical engineering, bioethics plays a crucial role in helping to ensure that innovations, such as OoC technology, human stem cell research, and organoids, are developed and applied in ways that respect human dignity, safety, and fairness.
Technological advances in these fields promise to revolutionize healthcare, from creating personalized treatments to understanding complex diseases and developing new therapies. However, as these innovations become more sophisticated, they also raise important ethical questions. How should we balance the potential benefits of these technologies with their risks? How do we ensure that research is conducted responsibly, with proper consent, safety, and transparency? And what role should the public play in shaping the future of biotechnology?
To explore these questions, we spoke with Dr. Jeremy Sugarman, an internationally recognized leader in bioethics, whose work has significantly impacted policy and ethics in many areas including stem cell research, HIV prevention, and global health. He is the Harvey M. Meyerhoff Professor of Bioethics and Medicine at Johns Hopkins University, US where he serves as a professor of medicine and health policy and management, as well as the deputy director of the Berman Institute of Bioethics.
The Ethical Questions Raised by Human Stem Cell Research, Organoids, and Organ-on-Chip Technology
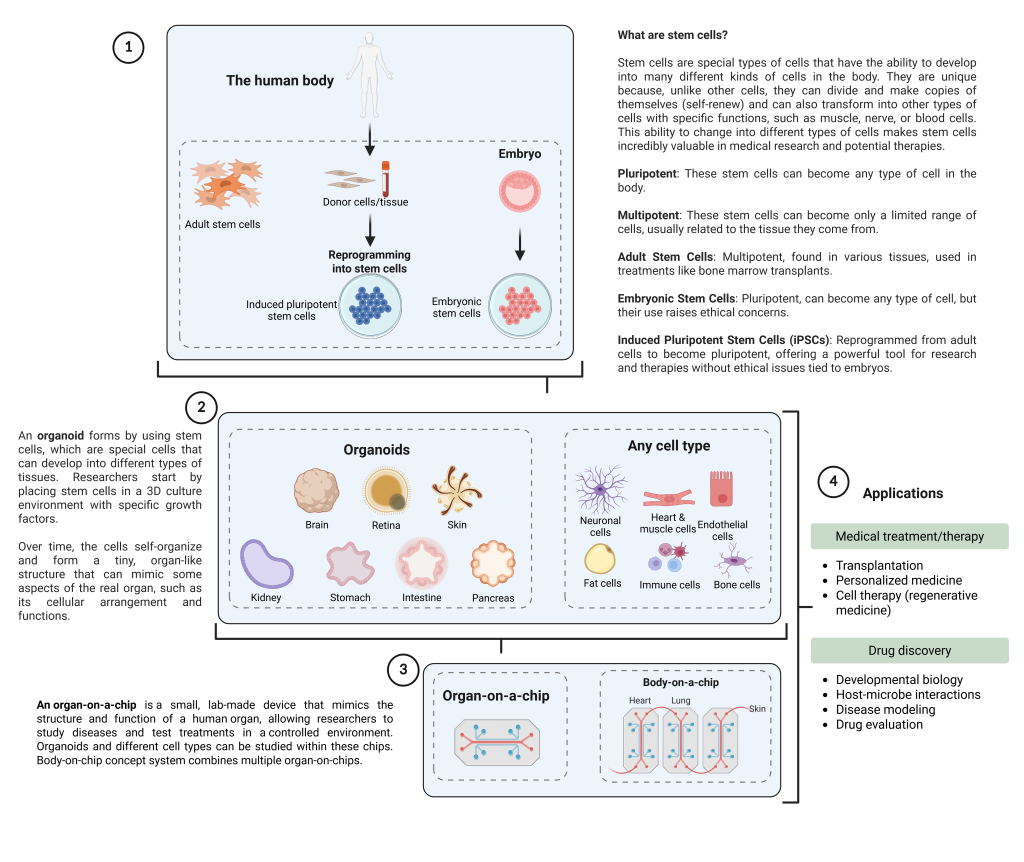
Emerging biotechnologies such as human pluripotent stem cell research, organoids, and OoC systems are revolutionizing medicine and science, but they also raise significant ethical concerns. Induced human pluripotent stem cells, which can be derived by reprogramming a person’s own cells and turned into various types of human tissue, have immense potential to treat diseases and model human biology. However, as Dr. Sugarman explains, the ethical issues surrounding human pluripotent stem cell-related research include the creation and use of human embryos, the safety of stem cell-based therapies, and concerns about commercialization. ” We need a lot more information to make sure interventions are safe and effective before we put them into people,” he says. He emphasizes that the slow and careful testing of stem cell-based therapies, such as those for Parkinson disease, is critical for ensuring patient safety and achieving clinical success.
Similarly, organoids—miniaturized, simplified versions of organs created from human stem cells—have gained significant attention for their ability to model human diseases. They offer a new way to study disease mechanisms and test potential treatments. However, the ethical implications of these technologies are not straightforward. While they represent cutting-edge advancements, they are not equivalent to fully functional human organs. “It’s just a partial model of function, which is really important to consider,” Dr. Sugarman points out. He warns against overhyping these technologies and urges researchers to be transparent about their limitations.
An OoC is a small, lab-made device that mimics the structure and function of a human organ or system, allowing researchers to study diseases and test treatments in a controlled environment. However, like organoids, they raise ethical questions about the manipulation and use of human tissues outside the body.
A critical part of the ethical framework around these technologies is informed consent. As Dr. Sugarman highlights, ensuring that proper consent is obtained from individuals who donate cells for research is essential, especially when there is potential for commercial use or when cells may be shared across research institutions. It is important that patients and donors understand how their cells will be used and that they are fully informed about the research being conducted.
Addressing Public Communication and Ethical Responsibility
One of the ethical challenges in bioethics involves communication about complex scientific advancements to the public. Researchers and institutions often face pressures to simplify their findings for broader audiences, especially when funding and public support are at stake. However, Dr. Sugarman emphasizes that scientists must be cautious to avoid sensationalizing their work. “The worst thing you can do is overinflate people’s hopes,” he cautions, explaining that hype can lead to disappointment and public disillusionment when promises fall short.
The ethical challenges regarding communication extend beyond just ensuring the accuracy of scientific reporting; it also involves engaging the public in meaningful conversations about the implications of these technologies. Dr. Sugarman advocates for a two-way communication model, where scientists actively listen to public concerns and values, rather than simply broadcasting information. “We need to elicit public values regarding this research,” he says. “By partnering with the public and co-creating ways to discuss these technologies, we can better navigate the ethical landscape.”

He further reflected on the complexity of translating stem cell research into clinical applications, stating, “Each step in the process, from research to clinical trials, must be approached with care. It’s not about rushing to deliver results, but rather ensuring the therapies are safe, effective, and ready for real-world use.” The ethical challenges surrounding stem cell research require careful consideration at every stage, from drug development to public communication, to avoid overhyping unproven treatments and misguiding the public.
Bioethics and the Future of Biotechnology
As biotechnology continues to evolve, the ethical challenges surrounding it will only become more complex. Dr. Sugarman believes that the future of bioethics lies in ensuring that researchers and clinicians are equipped to handle these challenges responsibly. He urges young scientists to learn about the ethical dimensions of their work and consider the implications of their discoveries. “The only way to be a truly good scientist is to learn about the ethical aspects of what you’re doing,” he says, adding that it’s essential for researchers to approach these issues thoughtfully and engage in open, informed dialogue about the potential consequences of their work.
While it’s impossible to predict exactly how bioethics will evolve alongside new technologies, Dr. Sugarman suggests that the best approach is to stay informed and engaged. By listening to a wide range of perspectives, scientists can better navigate the ethical questions that arise in rapidly advancing fields such as gene editing, AI, and organoid research.
Conclusion: Balancing Innovation with Ethical Responsibility
As we stand on the brink of scientific breakthroughs in fields such as human stem cell research, organoids, and OoC technologies, it is more important than ever to approach these advancements with caution and a strong ethical framework. Dr. Sugarman’s work reminds us that while these technologies hold incredible potential, they must be developed and applied in ways that prioritize human safety, dignity, and fairness. By fostering transparency, engaging with the public, and avoiding hype, we can ensure that these innovations lead to meaningful progress without compromising our ethical values.
As the field of bioethics continues to evolve, it will be the responsibility of researchers, policymakers, and the public to navigate the complex landscape of modern biotechnology. Only through careful consideration, open dialogue, and a commitment to ethical standards can we ensure that these powerful technologies are used for the greater good.

Dr. Jeremy Sugarman: An International Leader in Bioethics
Dr. Jeremy Sugarman is the Harvey M. Meyerhoff Professor of Bioethics and Medicine, as well as a professor of medicine and deputy director for medicine at the Berman Institute of Bioethics at Johns Hopkins University. A distinguished leader in the field of bioethics, Dr. Sugarman is known for his expertise in applying empirical methods to evaluate and address complex bioethical issues. His work has significantly advanced the ethical considerations surrounding stem cell research, HIV prevention, global health, and biomedical research oversight.
Dr. Sugarman, originally trained in internal medicine, developed an interest in bioethics early in his career, sparked by his experiences as an emergency medical technician. The ethical challenges in the case of Karen Ann Quinlan, a woman in a persistent vegetative state, further fueled his passion for the field. While in medical school, he organized a bioethics conference at Duke University and later conducted research at Oxford, Edinburgh, and Harvard. After residency, Dr. Sugarman earned a master’s in philosophy from Georgetown and a master’s in public health from Johns Hopkins, combining both theoretical and empirical approaches to bioethics.
With over 400 publications, including articles, book chapters, and influential books, Dr. Sugarman has been at the forefront of shaping ethical standards in medical and biomedical research. His contributions to bioethics are recognized worldwide, and he has served in critical advisory roles, including as a senior policy analyst for the White House Advisory Committee on Human Radiation Experiments and as a Senior Advisor to the Presidential Commission for the Study of Bioethical Issues. His guidance has been instrumental in developing policies and frameworks to ensure that research involving human subjects is conducted ethically, both in the U.S. and globally.
