At the CoEBoC, researchers from Computational Biophysics and Imaging Group (CBIG), in close collaboration with the Biomaterials and Tissue Engineering Group, developed a label-free quantitative imaging protocol based on Optical projection tomography (OPT) to analyze cell morphology and density in semi-transparent 3D hydrogels. The work shows the potential of an in-house-built OPT system to facilitate the development of tissue engineering scaffolds and organ-on-chip models.
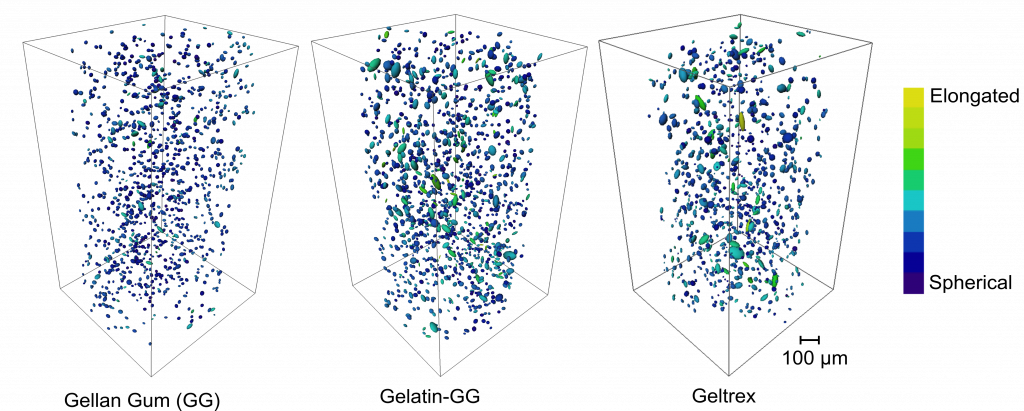
One of the aims of CoEBoC is to develop novel human tissue models with vasculature and innervation. For this new way to culture the tissues and to assess the cells in 3D is needed. Most imaging methods can only image samples either in limited depth or limited resolution. For example, a confocal microscope has been a valuable tool for high-resolution imaging of cells in three-dimensions (3D) but can only image samples at a depth up to roughly 300 µm. Besides, confocal imaging requires fluorescent staining of the structure or function. Thus, OPT is a valuable tool to be added besides the existing imaging systems to fill a gap in the imaging possibilities.
‘‘To advance the development of tissue engineering scaffolds for therapeutic or drug screening applications, we need to be able to image and analyze large volume samples with optimal resolution’’, says doctoral researcher Birhanu Belay. ‘‘And we need multimodal imaging tools that can help us with the paradigm shift from the traditional flat 2D to 3D cell culture models that are more representative of human tissue’’, Belay added.
In principle, OPT works similarly to X-ray micro-computed tomography, the sample is rotated through a series of angular positions, and two-dimensional projection images are captured at each orientation. But in OPT, visible light instead of X-rays are used to illuminate the samples. The standard filtered back-projection algorithm is used to reconstruct the 3D images from the 2D projections.
In the study, the capabilities of OPT for the analysis of cell responses in large volume hydrogel samples were shown. The 3D cell culturing, staining, imaging, and image processing techniques were optimized, and OPT was used to assess the cellular response in different hydrogel formulations. The cell morphological features were quantified to show how the cell responded to the 3D scaffold biomaterial. And through fluorescent staining of cells in 3D, cytocompatibility of the hydrogels was also evaluated. This is one of the main advantages of OPT over other techniques is its capability to provide images both in label-free bright-field and fluorescence modes.
This work is a game-changer, as most of the cell and tissue studies are currently described in a 2D environment, and cell culture in a 3D environment allows the more complex representation of biological processes. Further, OPT developed here opens new avenues for study in cell differentiation in a 3D environment.
A paper of the team´s breakthrough is published in Scientific Reports:
Belay, B., Koivisto, J.T., Parraga, J. et al. Optical projection tomography as a quantitative tool for analysis of cell morphology and density in 3D hydrogels. Sci Rep 11, 6538 (2021). https://doi.org/10.1038/s41598-021-85996-8
The research was funded by the Human Spare Parts program of Business Finland and CINOP Global, AAIT through the Nuffic-funded NICHE-Project NICHE/ETH/246, Wihuri Foundation, and the Finnish Cultural Foundation the Pirkanmaa Regional Fund.

